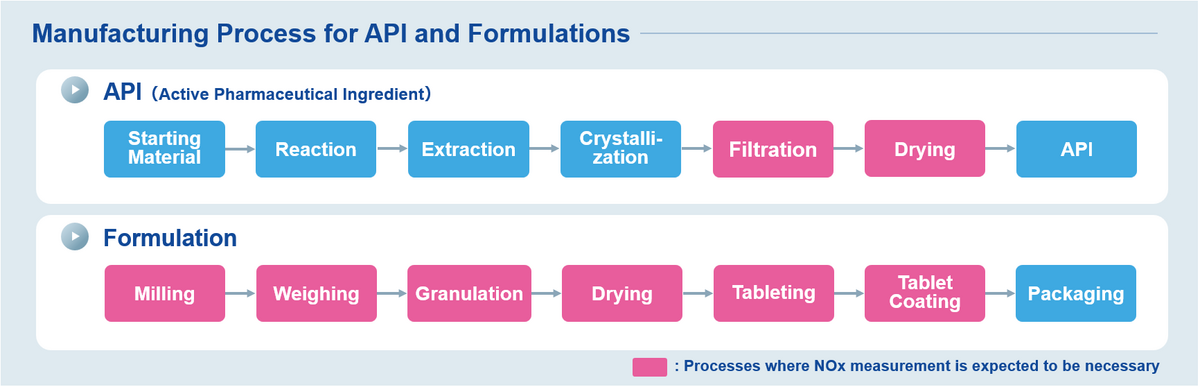
HORIBA's highly accurate trace gas analyzers can continuously monitor N compounds (NOx, NO2, NO) to help clarify the cause of nitrosamine formation during the process of API* and formulation of pharmaceutical manufacturing.
Recently, nitrosamines, which are likely carcinogenic to humans, have been detected globally in the pharmaceuticals such as Sartan class of drugs. The European Medicines Agency (EMA) and the United States Food and Drug Administration (FDA) recommend efforts to prevent the formulation of nitrosamines. Additionally, Japanese Ministry of Health, Labour and Welfare (MHLW) has notified pharmaceutical manufacturers and distributors to conduct self-inspections to assess the risk of nitrosamine contamination. The mechanism of nitrosamine formation is still under research, but one hypothesis suggests atmospheric N compounds could cause nitrosamine formation when ambient air is heated and used for drying formulation during the granulation process.
*API: Active Pharmaceutical Ingredient
Advantages of APNA-380
Nitrogen Oxides Monitor APNA-380
Nitrogen Oxides Monitor
Laser Scattering Particle Size Distribution Analyzer
Sie haben Fragen oder Wünsche? Nutzen Sie dieses Formular, um mit unseren Spezialisten in Kontakt zu treten.