Ions in Water, and Conductivity
Ions in Water, and Conductivity

We have so far dealt with Ohm's law and conductivity in general, and hope you understand the concept. You may wonder, however, what it has to do with the measurement of the conductivity of water--the real question from the beginning. So, we are now going into the main subject.
Flow of charge has been meant electric current till now. A metal, such as in an electric wire, contains a great number of free electrons. These electrons pass electric current from one to the next, just like a line of people forming a bucket brigade. Such a metal is called a conductor.
In the second place, let us introduce an ion conductor that its electric current is carried by ion, for example, electrolyte solution.
We will now discuss some of the new terms that have come up. When a certain substance is dissolved in liquid--water in the case of Twin--and if the liquid thus obtained can conduct electricity, such a liquid is called an electrolyte solution, and the dissolved substance is called an electrolyte. And each corpuscle that carries electricity is called an ion (a Greek word meaning wanderer).
Common table salt (NaCl) is an electrolyte, and when this is dissolved in water to form salt water, it becomes sodium ions (Na+) and chloride ions (Cl-), each of which is a corpuscle that conducts electricity.
Let's go back to conductivity. Conductivity is an index of how easy it is for electricity to flow. In water, it is the ions that pass electricity from one to the next. This means that the more Na+ and Cl- contained in water the more electricity is carried, and the higher the conductivity.
To sum up, if we know the conductivity of a sample of salt water, we can calculate just how salty the water is. (This is what happens in the salinity conversion to arrive at the value displayed by the Twin conductivity meter.)
Salinity (density of salt in salt water) and conductivity
Liquid temperature 25°C IEEE J.Ocean.Eng.,OE-5(1),3~8(1980).
| NaCl density (W / V) % | Conductivity (mS / cm) | NaCl density (W / V) % | Conductivity (mS / cm) |
|---|---|---|---|
| 0.1 | 2.0 | 1.1 | 19.2 |
| 0.2 | 3.9 | 1.2 | 20.8 |
| 0.3 | 5.7 | 1.3 | 22.4 |
| 0.4 | 7.5 | 1.4 | 24.0 |
| 0.5 | 9.2 | 1.5 | 25.6 |
| 0.6 | 10.9 | 1.6 | 27.1 |
| 0.7 | 12.6 | 1.7 | 28.6 |
| 0.8 | 14.3 | 1.8 | 30.1 |
| 0.9 | 16.0 | 1.9 | 31.6 |
| 1.0 | 17.6 | 2.0 | 33.0 |
Strong Electrolytes, Weak Electrolytes
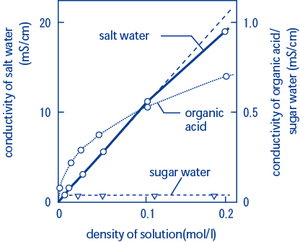
You now understand that we can determine the salinity of salt water by knowing its conductivity. Some of you may wonder whether sugar water can also be measured. Unfortunately, a conductivity meter cannot tell you the density of sugar in water. Although sugar is soluble in water, it does not form ions, which means that it is not an electrolyte. Only when ions are produced in water can the density of the dissolved substance be calculated from conductivity measured using a conductivity meter.
Like a human, an electrolyte has a variety of properties. Electrolytes can be broadly divided into strong electrolytes and weak electrolytes. Let's spend some time on this subject.
Strong electrolytes
Salt contains NaCl and KCl, which form electrolytes when dissolved in water, most of which become ions. The relationship between density and conductivity is nearly linear. As is seen in the diagram, however, unlike the low-density zone, the high-density zone does not show an increase in conductivity with a further increase in density. There comes a saturation point not unlike a traffic jam, where the ions act against each other, and this makes it hard for electricity to flow.
Weak electrolytes
In a very low density zone, conductivity has a linear relationship with density, as is seen with organic acids. Acetic acid solution is a good example. However, as density increases, the rate of ionization decreases. In the high-density zone, only part of the electrolyte is ionized, and the overcrowding causes most of the potential ions to remain dissolved in water as molecules.

A good couple and a bad couple
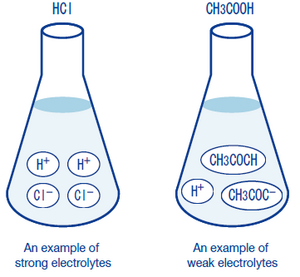
When CH3COOH is ionized, it becomes CH3COO- and H+, but since it is a weak electrolyte, most of the molecules remain CH3COOH. In other words, they are a good couple. When sodium acetate (CH3COONa) is ionized, it becomes the ions acetate CH3COO- and sodium ion Na+, but since it is a strong electrolyte, unlike acetate, it exhibits separation of most of its molecules. Unlike acetate, sodium acetate is like a bad couple.
About mol/l (moles per liter): The mol (symbol for the SI unit mole) is one of the chemical units we use for expressing the measured quantity of a substance. The number of atoms or molecules in one mol of a substance is equal to the Avogadro constant, which has a value of 6.022 X 1023. Therefore, the unit of density mol/l (moles per liter) indicates how much of a substance (in mol) is dissolved in 1 liter of a solution.

The History of Conductivity
Alessandro Volta was a physicist born in Italy in 1745. He became known in 1800 as the inventor of the first electric battery. Unlike the friction batteries known up to that time, the Volta battery provided continuous electric current, and was one of the great inventions of the century. This achievement by Volta paved the way for the likes of Georg Ohm, the German physicist who measured the conductivity of metals, and in 1827 discovered the now-famous Ohm's law.
Michael Faraday was born in 1791, the son of an English blacksmith. At age 13, he became a bookbinder's apprentice, which gave him access to many books. In 1833, he became an assistant to Professor Davies of the Royal Research Laboratory. He did prominent work in the fields of chemistry and physics, and in 1833, he conceived the law of electrolysis, and he envisioned ion as made of corpuscles that conveyed electricity in solution.
The conductivity of electrolytes was energetically measured by Friedrich Kohlrausch of Germany between 1869 and 1880. It is said that he started measuring conductivity as a means of obtaining ionic product. The Kohlrausch bridge, which he invented at that time for the purpose of measuring conductivity, is still well known today.

| A. Volta | (Italy) | 1745-1827 |
|---|---|---|
| G. Ohm | (Germany) | 1787-1854 |
| M. Faraday | (England) | 1791-1867 |
| F. Kohlrausch | (Germany) | 1840-1910 |
Next page Regulations Conductivity Meters