

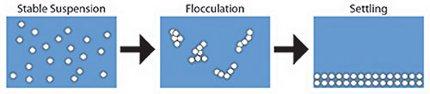
Suspended solids are a common impurity in wastewater from industrial and mining operations. Safety and aesthetics dictate that all water be essentially free of suspended matter. In order to meet requirements for water clarity for reuse or discharge, the suspended solids are often allowed to settle to the bottom or float (cream) to the top of large tanks. The duration of these processes is a strong function of the particle size, e.g. larger particles settle/cream faster. Thus, if particles can be made to aggregate, the settling process is shorter and therefore the treatment operation is faster and therefore less expensive.

Particle flocculation can be an energetically favored process; as particles flocculate, the total particle surface area is decreased and the system is more stable. However, flocculation cannot occur if particle collisions are suppressed by electrostatic interactions. That is, if two particles have the same charge (positive or negative), they will repel each other.
Aggregation is enhanced through the mechanism of charge neutralization; additives are used to reduce the magnitude of the particle surface charge (zeta potential) to zero. This increases the number of particle collisions and therefore the rate of flocculation. Naturally, this process needs to occur with minimal costs. This minimal cost requirement implies that the optimal amount of additive needs to be determined. One widely used class of such additives are termed coagulants and are typically inorganic salts with polyvalent ions. Unfortunately, addition of too much coagulant (overdosing) causes charge reversal resulting in restabilization of the suspended solids with a resultant deterioration in subsequent processing.
The zeta potential is related to the electrokinetic charge that exists at the surface of suspended particulate matter and so its measurement with the SZ-100V2 Nanoparticle Analyzer can be used to monitor the effect of coagulant addition. The results are then used to optimize the treatment process.
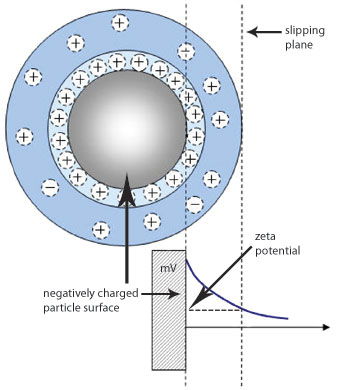
Zeta potential refers to the potential in the interfacial double layer (DL) at the location of the slipping plane versus a point in the bulk fluid away from the interface. In other words, zeta potential is the potential difference between the dispersion medium and the stationary layer of fluid attached to the dispersed particle. Since electrostatic interactions are important in many colloidal systems, this measure of electrostatic potential is important for controlling these systems. More details can be found in the zeta potential technology discussion.
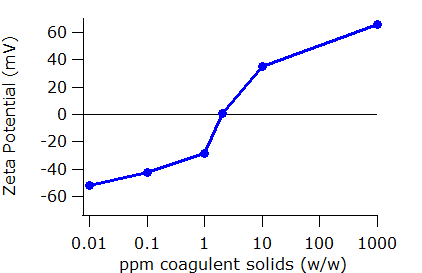
The SZ-100V2 Nanoparticle Analyzer can be used to monitor the effect of coagulant addition on particle surface charge. Example data showing the zeta potential of the wastewater suspension as a function of added coagulant is plotted below. As is common in this analysis, the added quantities are varied on a logarithmic scale.
As coagulant is added, the zeta potential approaches zero. There is an additional, and for this application, critical point in the data shown below. At one concentration of coagulant, the zeta potential reaches zero. This concentration is known as the iso-electric point. Then, with increasing coagulant concentration, the net charge on the particle becomes positive. Too much coagulant restabilizes the suspension but now with the opposite charge. The danger in adding too much coagulant is not merely the additional cost in reagents, but also that the treatment process will become ineffective.
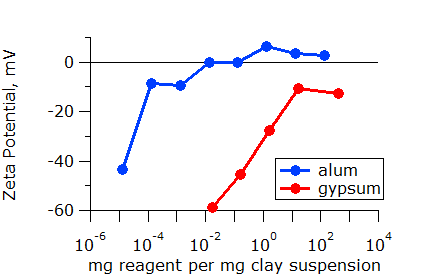
It is not always obvious which additive is best. More effective additives are often more expensive. Thus, knowing the required dose of each additive is critical to making the best decision. Once again, zeta potential measurements can make the choice clear. In the plot below, it is clear that about 100 times less gypsum is required than alum for this material. This is not unexpected as the trivalent aluminum ion (Al3+) in alum is known to be more effective than the divalent calcium ion (Ca2+) in gypsum.
In many processes, the nature of the waste evolves over time. Feed materials change, affecting the nature of the waste. Improvements to the upstream process may decrease the amount or type of waste. Thus, the behavior of waste will change over time and should be monitored. The frequency of measurement will depend on the stability of the process that feeds the waste stream.
The SZ-100V2 Nanoparticle Analyzer can measure the zeta potential of colloidal dispersions.
Learn how electrophoretic light scattering measures zeta potential.
Watch Webinar: Getting a Charge Out of Nanoparticles: Zeta Potential
Watch Webinar: Optimizing Wastewater Treatment using Zeta Potential
Watch Webinar from Brown & Caldwell: Integrated Characterization of Organic Matter for Water Treatment Optimization
Nanoparticle Analyzer
Do you have any questions or requests? Use this form to contact our specialists.