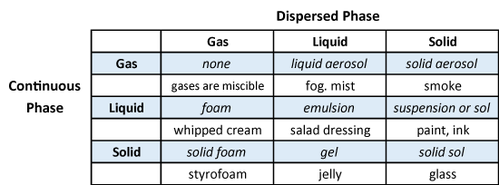
A colloid is typically a two phase system consisting of a continuous phase (the dispersion medium) and dispersed phase (the particles or emulsion droplets). The particle size of the dispersed phase typically ranges from 1 nanometer to 1 micrometer. Examples of colloidal dispersions include solid/liquid (suspensions), liquid/liquid (emulsions), and gas/liquid (foams). A more complete range of colloidal dispersions is shown in the table below.
As particle size decreases, surface area increases as a function of total volume. In the colloidal size range there is much interest in particle-particle interactions. Most colloidal commercial products are designed to remain in a stable condition for a defined shelf life. Milk is an example where homogenization is used to reduce droplet size to delay the onset of phase separation (i.e., creaming with the fat rising to the surface). Commercial suspensions may be formulated to keep particles in suspension without sedimenting to the bottom.
Stabilization serves to protect colloids from aggregation and/or phase separation. The two main mechanisms for colloid stabilization involve steric and electrostatic modifications. Electrostatic stabilization is based on the mutual repulsion of like electrical charges. By altering the surface chemistry to induce a charge on the surface of particles it is possible to enhance the stability of the colloidal dispersion.
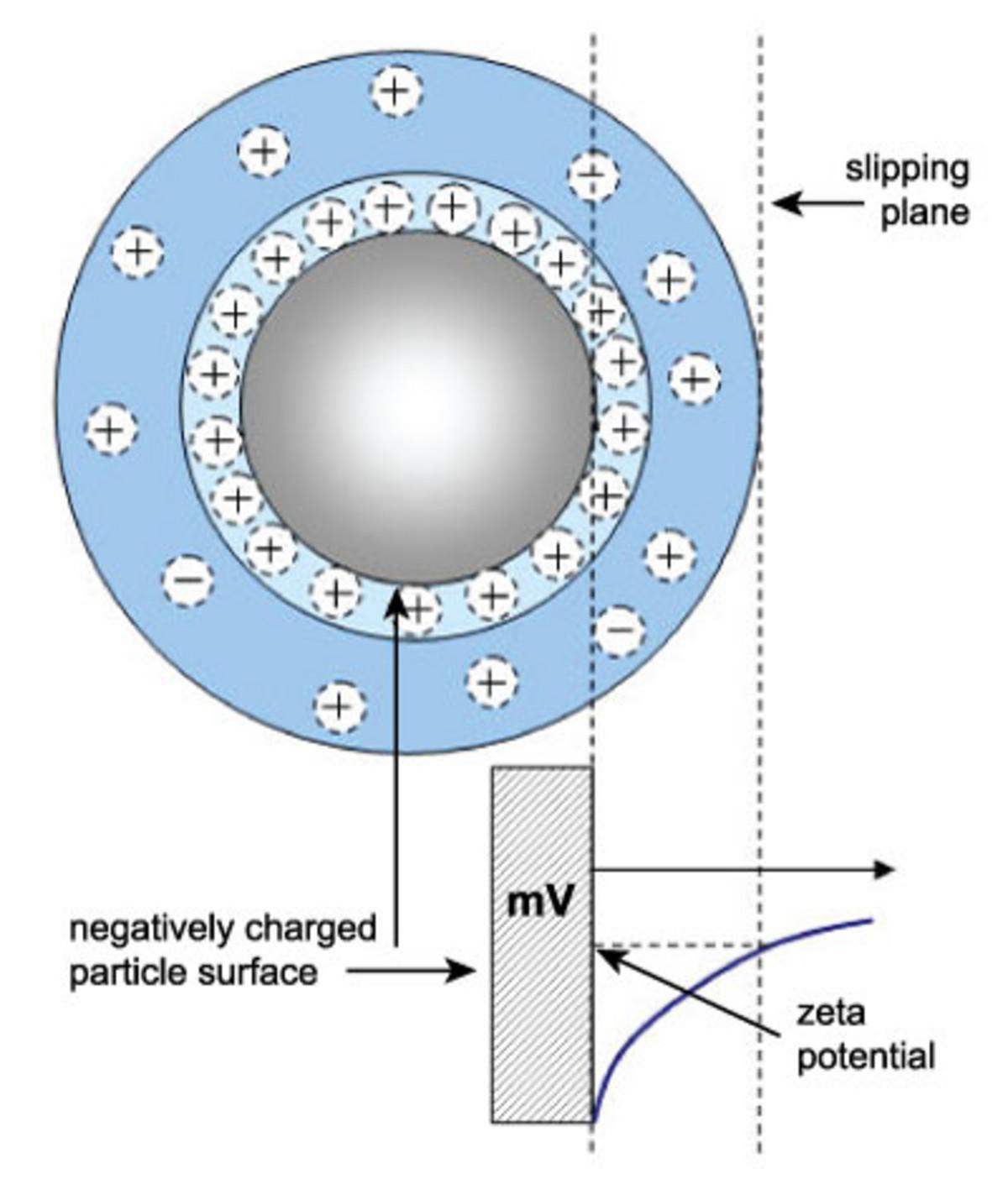
Zeta potential refers to the potential in the interfacial double layer (DL) at the location of the slipping plane versus a point in the bulk fluid away from the interface. In other words, zeta potential is the potential difference between the dispersion medium and the stationary layer of fluid attached to the dispersed particle. A classic example of colloid chemistry is to measure zeta potential vs. pH to determine the conditions where the zeta potential reaches zero, known as the isoelectric point.
Scientists working to improve colloidal stability measure particle size, zeta potential, or both. Various techniques are now capable of measuring particle size into the colloidal region including dynamic light scattering (DLS) and laser diffraction. The SZ-100V2 nanoPartica DLS system can measure particle size and zeta potential of colloidal dispersions and has the option of an automatic titrator for zeta potential vs. pH studies. The LA-960V2 laser diffraction particle size analyzer is the best choice when particles above 1 micron may also be present in the particle system.
Watch our webinar: "Getting a Charge Out of Nanoparticles: Zeta Potential"
Laser Scattering Particle Size Distribution Analyzer
Nanoparticle Analyzer
Simultaneous Multi-Laser Nanoparticle Tracking Analysis (NTA)
Do you have any questions or requests? Use this form to contact our specialists.