

Battery technology is improving, keeping up with the demand for more portable devices and the desire for better power storage for longer periods between charging and changing batteries. Improved performance requires greater control of the materials used and their physical properties including the particle size distribution. In this study, the LA-960 laser diffraction particle size analyzer was used to perform particle size distribution measurements on various materials used in the creation of lithium ion batteries.
Batteries containing lithium include disposable (primary) lithium batteries, rechargeable (secondary) lithium ion batteries, and rechargeable lithium titanate batteries. Disposable lithium batteries have lithium metal or lithium compounds as the anode. Rechargeable lithium ion batteries use an intercalated lithium compound as the electrode material. A lithium titanate battery is a modified lithium-ion battery that uses lithium titanate nanocrystals on the surface on the anode.

A partial list of cathode and anode materials used in lithium batteries is shown below:
The particle size distribution (PSD) of the materials used to make these batteries is tested in both R&D environments and in QC for product acceptance since a PSD specification typically exists for the material. Particle size influences both capacity and coulomb efficiency. Reducing the PSD will increase the specific surface area, changing important battery characteristics, but this also changes the size of the voids between electrode particles, reducing battery capacity.
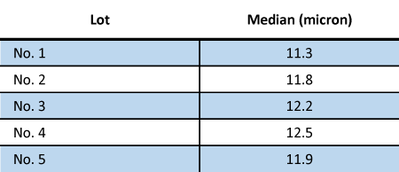
Lithium cobalt oxide (LiCoO2) has served as the archetypical cathode material for secondary Li-ion batteries since the 1980’s. Five different lots of lithium cobalt oxide powder to be used as cathode material were analyzed on the LA-960 Particle Size Analyzer. The powder was dispersed in water containing 0.2% sodium hexametaphosphate. This was standard pass/fail testing to determine if the material met the incoming material particle size specification. The results from the different lots are shown in Figure 1 and Table 1.
Figure 1: Five lots of LiCoO2 powder
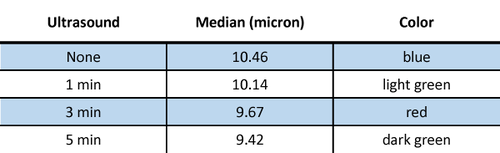
During the method development for dispersing lithium manganese oxide (LiMn2O4) the effect of applying ultrasound was investigated. The powder was dispersed in DI water containing 0.2% sodium hexametaphosphate. The sample was analyzed without ultrasound, and then with 1, 3, and 5 minutes of ultrasound. The results for these measurements are shown in Figure and Table 2. The optimal time of ultrasound was determined to be 3 minutes.
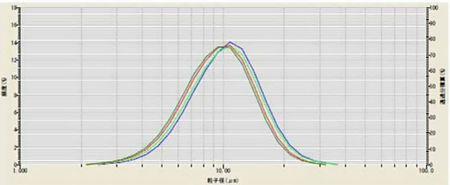
Figure 2: The effect of ultrasound on LiMn2O4
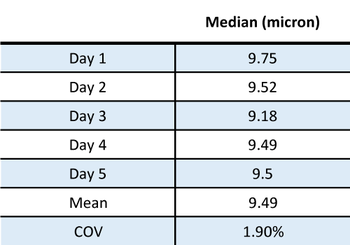
Once it was determined that 3 minutes ultrasound would be used in this method, the method was repeated on the same sample each day for 5 days to test reproducibility. These results are shown in Figure and Table 3.
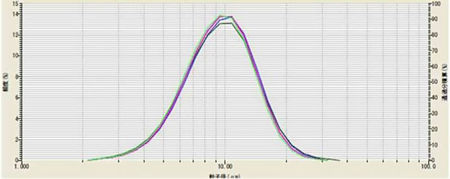
Figure 3 and Table 3: Method validation testing on multiple days of LiMn2O4
Lithium titanate (Li2TiO3) is often used as the anode material for fast recharging lithium titanate batteries. During the method development for dispersing lithium titanate powder in liquid the effect of applying ultrasound was investigated. The powder was dispersed in DI water containing 0.2% sodium hexametaphosphate. The sample was analyzed without ultrasound, and with 3 minutes of ultrasound. The results for these measurements are shown in Figure 4. This dispersion of this sample did not improve with the addition of ultrasound, so it was not used in the method.
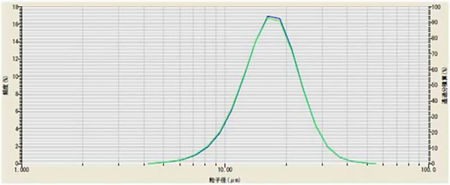
Figure 4: Li2TiO3 with (green) and without (blue) ultrasound
Two samples of lithium titanate from different suppliers were then compared by measuring the PSD of both products on the LA-960. The powders were dispersed in DI water containing 0.2% phosphoric acid and 0.2% sodium hexametaphosphate. The comparison of Sample A (mean = 6.33 µm) to Sample B (mean = 16.7 µm) is shown in Figure 5.
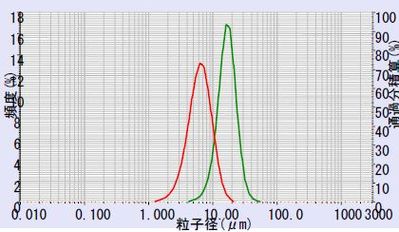
Figure 5: Li2TiO3 sample A (red) vs. Sample B (green)
Two samples (LiMn2O4 and Li2TiO3) were analyzed ten times to quantify the reproducibility of the LA-960. The results are shown in Figure 6.
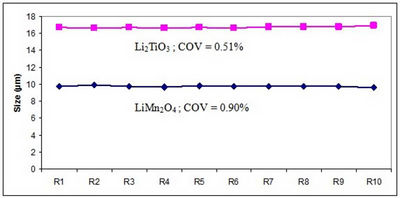
Figure 6: Reproducibility over 10 results for LiMn2O4 and Li2TiO3
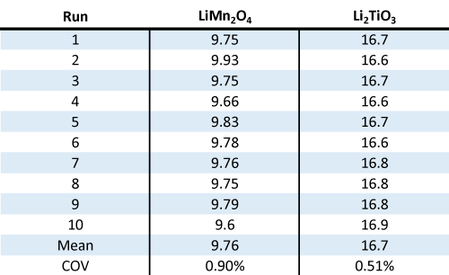
Note that the COV values are very low, indicating a good method and the high performance level of the LA-960 particle size analyzer.
Companies with multiple sites often need to compare data from the different laboratories. Samples of lithium manganese oxide and lithium titanate were analyzed on two different LA-960 systems in order to quantify the instrument to instrument agreement. The results from the comparison are shown below in Figure 7.
Figure 7: Instrument to instrument agreement for LiMn2O4 and Li2TiO3 on two different LA-960 systems

The LA-960 proved to have exceptional reproducibility and agreement between systems when measuring the PSD of several battery materials. This performance level has been proven to many manufacturers of battery materials such as the lithium compounds shown in this application note, leading to multiple sales to suppliers and users around the world.
Acknowledgement: Many thanks to the HORIBA Applications Center in Kyoto for generating the excellent data shown in this application note.
Laser Scattering Particle Size Distribution Analyzer
Do you have any questions or requests? Use this form to contact our specialists.