Detector (Reference electrode, Temperature-compensation electrode, Combination electrode)
Reference electrode
A reference electrode is used in combination with a glass electrode so that the difference in potential generated between it and the glass electrode can be measured. It also indicates a known potential irrespective of the pH of the solution.
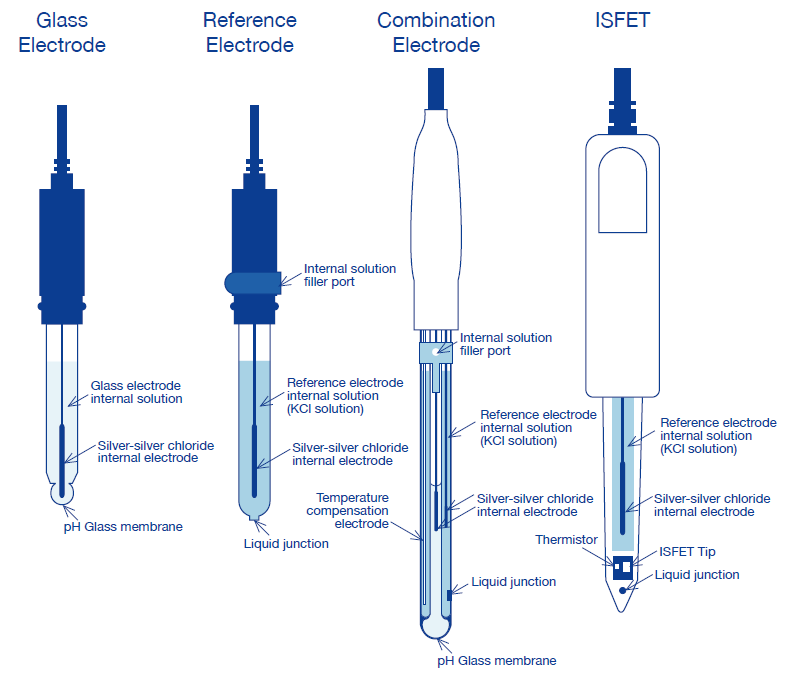
As shown in the figure, it consists of a liquid junction, internal solution, internal solution filler port, a tube to support the reference electrode, the internal solution of the reference electrode, an internal electrode and an electrode lead wire. In most cases, a silver chloride electrode or mercurous chloride electrode is used as the internal electrode, and potassium chloride is used as the internal solution.
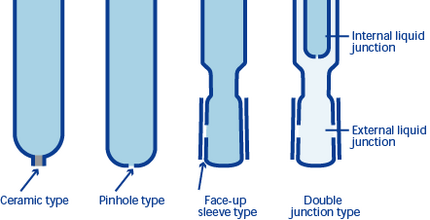
The liquid junction contacts the test solution and the internal solution. This is roughly classified into four types: (1) the pinhole type, which has a hole a few dozen microns in diameter, (2) the sleeve type, which has a petticoat facing upward, (3) the ceramic type, which contacts foreign material, and (4) the fiber type. The pinhole liquid junction has the advantage of very small loss of the internal solution; however, it tends to generate liquid potential. The sleeve liquid junction is easy to clean, but loss of internal solution is higher. The ceramic and fiber liquid junctions exhibit less loss of internal solution, but a problem with adherence of test solution. In light of these advantages and disadvantages, a double-junction type was developed by combining two types of junctions.
![]()
Liquid Junction potential
Liquid junction potential means how much potential is generated at the liquid junction. It varies depending on the type and temperature of the solution and the structure of the liquid junction. When contact is made with solutions of different compositions, the activities of positive and negative ions of each solution are different and diffuse at the contact surface of both liquids. As factors such as ion size may differ between the two liquids, the movement speed of diffusion differs. Therefore, we assume that as the diffusion progresses, a separation of charge is caused at the contact surface of the two liquids, generating a difference in potentials. This difference in potentials decreases the speed of fast-moving ions and increases the speed of slow-moving ions. Therefore, the speed of movement of negative and positive ions becomes equal at the contact surface. The difference in potentials at the contact surface of the two liquids whose speeds of ion movement are equal is called liquid junction potential. A large liquid junction potential causes fluctuations in the reference voltage of reference electrodes, causing serious inaccuracies in measurement in both the hydrogen electrode and glass electrode methods.
Temperature-compensation electrode
A temperature-compensation electrode is needed, because the electromotive force generated at the glass electrode varies depending on the temperature of solution.
Temperature compensation means compensating for the variation of electromotive force due to a variation in temperature. What needs to be understood thoroughly here is that a variation of pH values due to temperature has nothing to do with compensation for temperature.
Therefore, one must record the temperature of a solution along with the pH value, even if using a pH meter that automatically compensates for temperature. Otherwise, the measured values may become meaningless.
Combination electrode
With the combination electrode, the glass electrode, reference electrode, and temperature-compensation electrode are all integrated into one unit.This enables pH measurement only by immersing a single electrode into the sample solution. It is easy to use and convenient when cleaning and calibrating with standard solution.
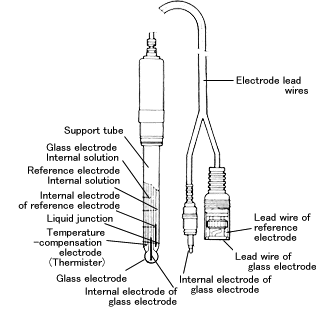
Structure of Combination Electrode
Electrode Lineup