Ways of Measuring pH

The methods for measuring pH fall roughly into the following four categories:
- Indicator methods
- Metal-electrode methods (including the hydrogen-electrode method, quinhydron-electrode method and antimony-electrode method)
- Glass-electrode methods
- Semiconductor sensor methods
Then, each measuring method is explained briefly.
(1) Measuring pH Using an Indicator
This category basically includes two methods: One involves comparing the standard color corresponding to a known pH with the color of an indicator immersed in the test liquid using buffer solution. The other method involves preparing pH test paper which is soaked in the indicator, then immersing the paper in the test liquid and comparing its color with the standard color. This method is simple, but prone to error. A high degree of accuracy cannot be expected.
* Various errors include;
- Error due to high salt concentration in the test liquid
- Error due to the temperature of the test liquid
- Error due to organic substances in the test liquid
The indicator method cannot measure the pH of high-purity water, since the influence of the indicator itself is too large.
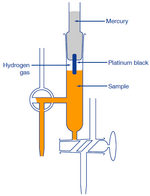
(2) Hydrogen-Electrode Method
A hydrogen electrode is made by adding platinum black to platinum wire or a platinum plate. It is immersed in the test solution and an electric charge is applied to the solution and the solution is saturated with hydrogen gas.The electrode potential is measured between platinum black electrode and silver chloride electrode. This potential is inversely proportional to pH of the solution.
The hydrogen-electrode method is a standard among the various methods for measuring pH. The values derived using other methods become trustworthy only when they match those measured using hydrogen electrode method.
However, this method is not appropriate for daily use because of the effort and expense involved, with the inconvenience of handling hydrogen gas and great influence of highly oxidizing or reducing substances in the test solution.
(3) Quinhydron-Electrode Method
When quinhydrone is added to a solution, it separates into hydroquinone and quinone.
Because quinone’s solubility varies depending on the pH value of the solution, pH can be determined from the voltage between a platinum and reference electrode.
Although this method is simple, it is seldom used today, because it does not work when oxidizing or reducing substances are involved, or when the test solution has a pH above 8 or 9.
Note: Quinhydron solution of a certain pH is sometimes used to check whether an ORP meter is operating normally. The principle of the quinhydron electrode is applied in such a case.
(4) Antimony-Electrode Method
This method involves immersing the tip of a polished antimony rod into a test solution, also immersing a reference electrode, and measuring pH from the difference in potential between them. This method was once widely used because the apparatus is sturdy and easy to handle. However, its application is now quite limited because results vary depending on the degree of polish of the electrode, and reproducibility is low.
Note: This method is now used only in cases where a high degree of accuracy is not required (only for industrial use) and the test solution contains F-.
(5) Glass-Electrode Method
The glass electrode method uses two electrodes, a glass electrode and reference electrode, to determine the pH of a solution by measuring the voltage (potential) between them.
This method is the one most commonly used for pH measurement, since the potential quickly reaches equilibrium and shows good reproducibility, and because the method can be used on various types of solutions, with oxidizing or reducing substances having very little impact on the result.
The glass electrode method is widely used, not only in industry but also in many other fields.
In its “Methods of pH Measurement” section, JIS states, “Since measurement using a hydrogen electrode, as described in the definition section, is not necessarily appropriate, measurement using a glass electrode is recommended for industrial pH measurement.”
(6)Semiconductor sensor methods
The semiconductor pH sensor, whose development started around 1970, replaces a glass electrode with a semiconductor chip. This sensor, known as an ion sensitive field effect transistor (ISFET), is not only resistant to damage but also easily miniaturized. Miniaturization allows the use of smaller amounts of sample for measurement, and makes it possible to perform measurements in very small spaces and on solid state surfaces. This sensor promises useful applications in measurement in the fields of biology and medicine.

pH imaging
This is a measurement method in which pH is determined at multiple points and the results displayed as an image. It has been attracting attention as a technology for visualizing pH distributions.
Historically, the method mentioned in Section (1), which combines pH indicators and microscopes, has been used to measure the pH inside cells. However, the invention of pH-sensitive dyes and proteins as a replacement for pH indicators has aided investigation of pH distribution in cells. In recent years, there have been examples of reports in which the multiple arrays of semiconductor pH sensors described in Section (6) have been used for pH imaging.
Reference: Satoshi Nomura, Bunseki, vol. 18, p468, 2011.
Relation page The Basis of pH