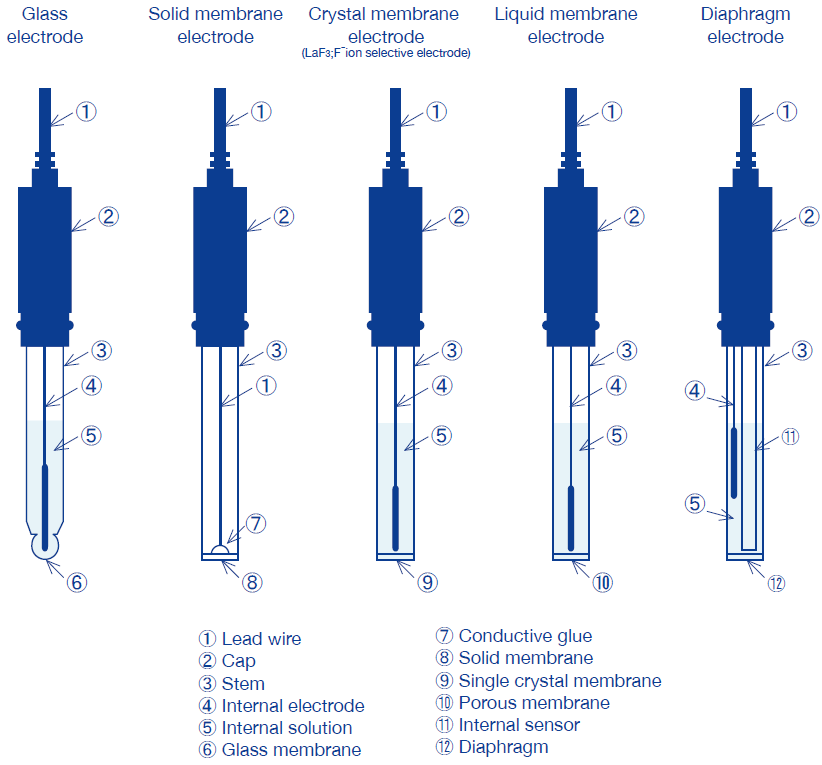
Categorization of Ion electrodes by response membrane
Ion selective electrodes are categorized into the five types shown below, depending on response membrane.
Electrodes have a different internal resistance depending on their response membrane, and also require different measurement methods, electrode storage, and electrode maintenance. Please read the precautions attached to each electrode carefully.
Type | Electrode structure | Typical ions measured |
|---|---|---|
Glass electrode | Response membrane consists of a glass thin membrane. | Na+, H+ |
Solid membrane electrode | Response membrane is composed of a single crystal of—or pressure-molded powder consisting primarily of— poorly-soluble metallic salt. | Cl-, Br-, I-, SCN-, CN-, S2-, Ag+, Pb2+, Cu2+, Cd2+ |
F- electrodes have a response membrane composed of a single crystal of lanthanum fluoride LaF3, and have a neutral internal solution containing F- ions. | F- | |
Liquid membrane electrode | Electrode has a response membrane which consists of a porous membrane or macromolecular material that acts as a support for polar organic solvent containing dissolved liquid ionic-exchanger. Also known as a plastic solidification membrane electrode. | NO3-, Ca2+, K+ |
Diaphragm electrode | Electrode is composed of a stem into which an internal pH glass and reference electrode have been inserted, and is filled internally with liquid and coated with a diaphragm. | NH3 |
Next page Selectivity coefficient